Magnesium Bromide + Chlorine Gas . instructions on balancing chemical equations: cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. Enter an equation of a chemical reaction and click 'balance'. br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. this equation represents the reaction that takes place when sodium metal is placed in water. This is a single replacement (double replacement) reaction involving halogens instead of metals. Mgbr2 + cl2 → mgcl2 + br2. mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. the balanced equation for the reaction of magnesium bromide and chlorine is mgbr2 + cl2 = br2 + mgcl2. in this video we'll balance the equation mg + cl2 = mgcl2. The solid sodium reacts with liquid.
from us.metoree.com
the balanced equation for the reaction of magnesium bromide and chlorine is mgbr2 + cl2 = br2 + mgcl2. this equation represents the reaction that takes place when sodium metal is placed in water. in this video we'll balance the equation mg + cl2 = mgcl2. Enter an equation of a chemical reaction and click 'balance'. Mgbr2 + cl2 → mgcl2 + br2. The solid sodium reacts with liquid. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. This is a single replacement (double replacement) reaction involving halogens instead of metals.
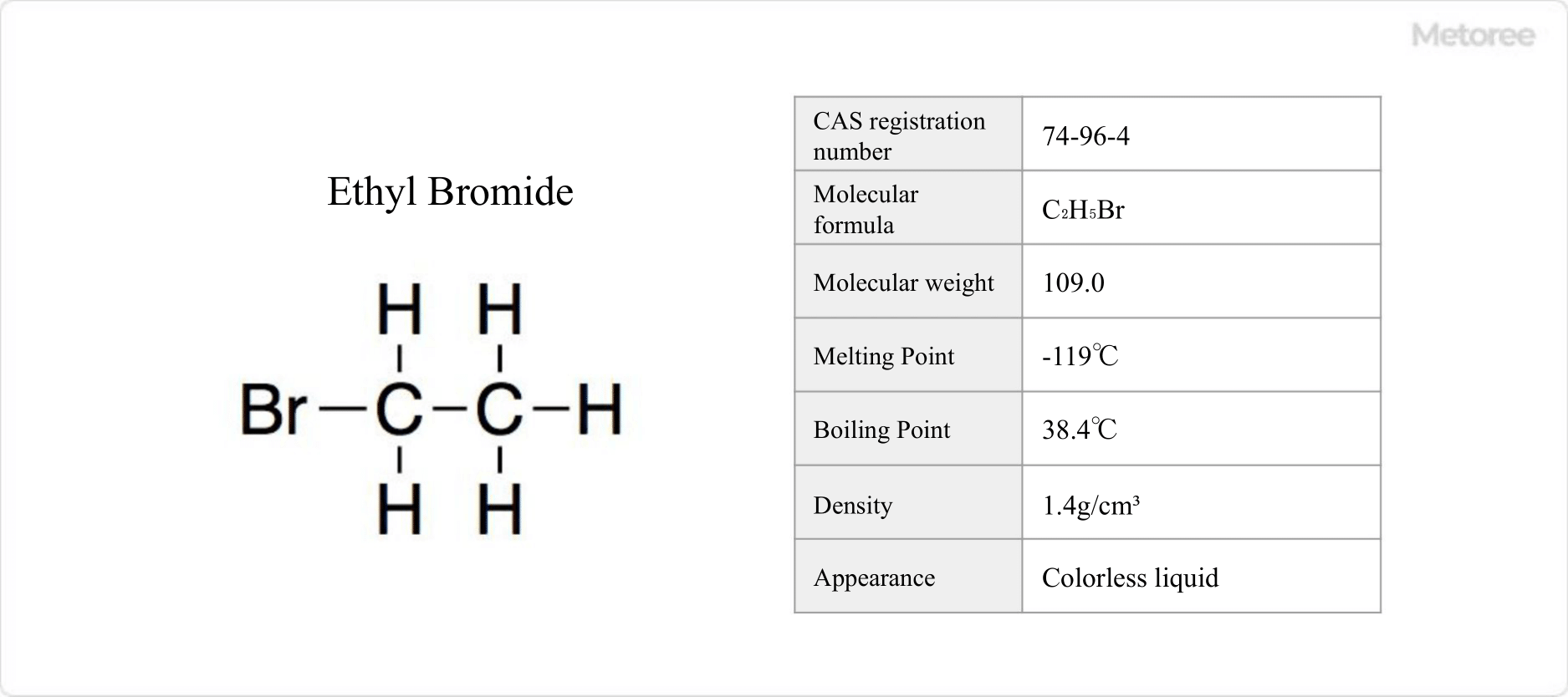
41 Ethyl Bromide Manufacturers in 2024 Metoree
Magnesium Bromide + Chlorine Gas cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. Enter an equation of a chemical reaction and click 'balance'. This is a single replacement (double replacement) reaction involving halogens instead of metals. this equation represents the reaction that takes place when sodium metal is placed in water. The solid sodium reacts with liquid. instructions on balancing chemical equations: Mgbr2 + cl2 → mgcl2 + br2. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. the balanced equation for the reaction of magnesium bromide and chlorine is mgbr2 + cl2 = br2 + mgcl2. in this video we'll balance the equation mg + cl2 = mgcl2. mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Magnesium Bromide + Chlorine Gas This is a single replacement (double replacement) reaction involving halogens instead of metals. mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. instructions on balancing chemical equations: cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl]. Magnesium Bromide + Chlorine Gas.
From sielc.com
Vinyl bromide SIELC Technologies Magnesium Bromide + Chlorine Gas br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. instructions on balancing chemical equations: Mgbr2 + cl2 → mgcl2 + br2. This is a single replacement (double replacement) reaction involving halogens instead of metals. Enter an equation of a chemical reaction and click 'balance'. The solid sodium. Magnesium Bromide + Chlorine Gas.
From hamptonresearch.com
Hampton Research Magnesium Bromide + Chlorine Gas This is a single replacement (double replacement) reaction involving halogens instead of metals. this equation represents the reaction that takes place when sodium metal is placed in water. Enter an equation of a chemical reaction and click 'balance'. mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide.. Magnesium Bromide + Chlorine Gas.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Magnesium Bromide + Chlorine Gas Enter an equation of a chemical reaction and click 'balance'. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. The solid sodium reacts with liquid. br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide.. Magnesium Bromide + Chlorine Gas.
From www.indiamart.com
Sodium Bromide 45, Bromide salt of sodium, NaBr, 7647156, Sedoneural Magnesium Bromide + Chlorine Gas mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. in this video we'll balance the equation mg + cl2 = mgcl2. br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. the balanced equation for. Magnesium Bromide + Chlorine Gas.
From www.solcohealthcare.com
Rocuronium Bromide Injection Solco Healthcare Magnesium Bromide + Chlorine Gas in this video we'll balance the equation mg + cl2 = mgcl2. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. Mgbr2 + cl2 → mgcl2 + br2. Enter an equation of a chemical reaction and click 'balance'. the balanced equation for the reaction. Magnesium Bromide + Chlorine Gas.
From www.scientificlabs.co.uk
Ethidium bromide, BioReagent, E76375G SIGMA ALDRICH SLS Magnesium Bromide + Chlorine Gas br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. the balanced equation for the reaction of magnesium bromide and chlorine is mgbr2 + cl2 = br2 + mgcl2. instructions on balancing chemical equations: The solid sodium reacts with liquid. in this video we'll balance the. Magnesium Bromide + Chlorine Gas.
From www.youtube.com
Preparation of Acetic Acid from Grignard ReagentDry IceMethyl Magnesium Bromide + Chlorine Gas this equation represents the reaction that takes place when sodium metal is placed in water. Enter an equation of a chemical reaction and click 'balance'. instructions on balancing chemical equations: cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. the balanced equation for. Magnesium Bromide + Chlorine Gas.
From www.indiamart.com
BioTech Grade Powder Sodium Bromide for Petroleum Industry, for Magnesium Bromide + Chlorine Gas this equation represents the reaction that takes place when sodium metal is placed in water. This is a single replacement (double replacement) reaction involving halogens instead of metals. Mgbr2 + cl2 → mgcl2 + br2. br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. the balanced. Magnesium Bromide + Chlorine Gas.
From www.chemkits.eu
Potassium bromide, 98.5, 7758023 Magnesium Bromide + Chlorine Gas The solid sodium reacts with liquid. mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. Mgbr2 + cl2 → mgcl2 + br2. instructions on balancing chemical equations: this equation represents the reaction that takes place when sodium metal is placed in water. the balanced equation. Magnesium Bromide + Chlorine Gas.
From www.alamy.com
Bubbling Chlorine Gas into Sodium Bromide to Yield Bromine Stock Photo Magnesium Bromide + Chlorine Gas in this video we'll balance the equation mg + cl2 = mgcl2. br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. The solid sodium reacts with liquid. This is a single replacement (double replacement) reaction involving halogens instead of metals. Enter an equation of a chemical reaction. Magnesium Bromide + Chlorine Gas.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Magnesium Bromide + Chlorine Gas in this video we'll balance the equation mg + cl2 = mgcl2. this equation represents the reaction that takes place when sodium metal is placed in water. br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. mgbr2 + cl = mgcl2 + br is a. Magnesium Bromide + Chlorine Gas.
From www.vectorstock.com
Ch3br methyl bromide molecule Royalty Free Vector Image Magnesium Bromide + Chlorine Gas this equation represents the reaction that takes place when sodium metal is placed in water. mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. The solid sodium reacts with liquid. br2mg + cl = cl2mg + br is a single displacement (substitution) reaction where one mole. Magnesium Bromide + Chlorine Gas.
From nsilabsolutions.com
Bromide CRM IS019 NSI Lab Solutions Magnesium Bromide + Chlorine Gas this equation represents the reaction that takes place when sodium metal is placed in water. instructions on balancing chemical equations: cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. Enter an equation of a chemical reaction and click 'balance'. mgbr2 + cl =. Magnesium Bromide + Chlorine Gas.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Magnesium Bromide + Chlorine Gas cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. in this video we'll balance the equation mg + cl2 = mgcl2. mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. This is a. Magnesium Bromide + Chlorine Gas.
From www.grainger.com
7758023, 119, Potassium Bromide, Crystal, Reagent, ACS 6MMU0P1220 Magnesium Bromide + Chlorine Gas mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. Enter an equation of a chemical reaction and click 'balance'. The solid sodium reacts with liquid.. Magnesium Bromide + Chlorine Gas.
From www.firsthope.co.in
Methantheline Bromide Chemical Structure, Mechanism of Action, Uses Magnesium Bromide + Chlorine Gas mgbr2 + cl = mgcl2 + br is a single displacement (substitution) reaction where one mole of aqueous magnesium bromide. Enter an equation of a chemical reaction and click 'balance'. cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. the balanced equation for the. Magnesium Bromide + Chlorine Gas.
From mavericksrpatterson.blogspot.com
Chemical Formula of Bromide MavericksrPatterson Magnesium Bromide + Chlorine Gas cl + mgbr2 = mgcl2 + br2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. The solid sodium reacts with liquid. instructions on balancing chemical equations: in this video we'll balance the equation mg + cl2 = mgcl2. the balanced equation for the reaction of magnesium bromide and chlorine. Magnesium Bromide + Chlorine Gas.